Percutaneous Irreversible Electroporation of Surgically Unresectable Pancreatic Cancer:A Case Report
A 76-year-old African American male diagnosed with stage III
(tumor/node/metastasis stages T4N0M0) unresectable pancreatic cancer secondary
to vascular invasion was referred for percutaneous IRE after he refused
chemotherapy or radiation. Computed tomography (CT) imaging (Fig 1) revealed a 4.1 *4.1 *3.5-cm
mass with encasement of the celiac axis and origin of the superior mesenteric
artery and occlusion of the extrahepatic portal vein and superior mesenteric vein.
A whole-body staging CT scan demonstrated
no metastatic disease.
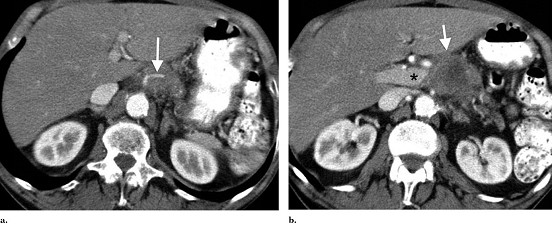
Figure 1. Staging CT scan. (a) Superior aspect of low attenuation pancreatic carcinoma. Note occlusion of the splenic artery (arrow) within the mass. (b) Carcinoma (arrow) involves the portal vein confluence (asterisk) and superior mesenteric artery.
Percutaneous ablation was planned and performed as two ablative sessions
to avoid the need for more than six probes
to be placed at once. The patient was administered general anesthesia, and four
15-cm monopolar probes (Na- noknife; AngioDynamics,
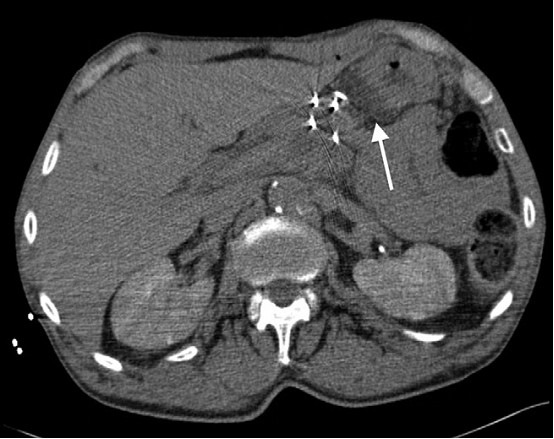
Figure 2. IRE needle placement. Probes placed from anterior approach through the mass. A 22-g needle is also demonstrated between the pancreas and stomach for hydrodissection (arrow).
Six vectors (Fig 3) for pulse delivery were chosen with maximum and minimum interprobe distance
of 2.3 and 1.4 cm, respectively. All pulses were administered in the absolute
refractory period with use of electrocardiographic synchronization (AccuSync,
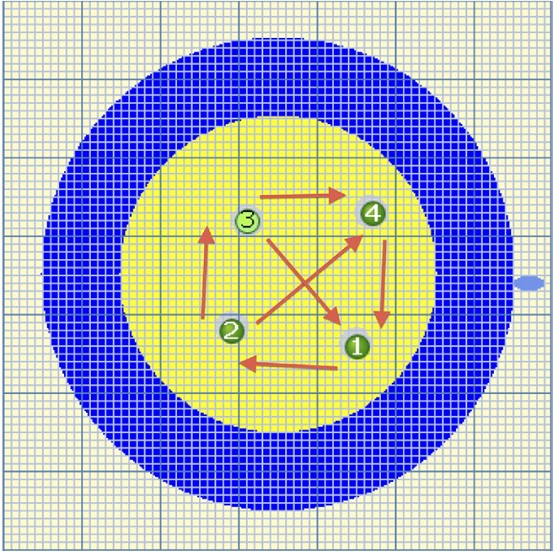
Figure 3. Graphic from
Nanoknife software amended (arrows) to
illustrate six vectors of pulse delivery.
After 2 weeks, the patient underwent a second IRE procedure targeting the untreated medial
portion of the tumor, which was not treated during the initial intervention. The
second ablation was performed by using a similar protocol with three probes.
The probes were directed from lateral to medial and positioned under
At 2-week clinical follow-up, the patient had mild, intermittent pain,
without fatigue, fever, or other symptoms.Contrast-enhanced magnetic resonance
(MR) imaging (Fig 4) was performed within 24 hours of each ablation and 30 days
after the second ablation, and demonstrated an absence of enhancement within
the expected ablation zone. Vasculature with the ablation zone, specifically
the splenic artery and superior mesenteric artery, remained patent and
unchanged from its preoperative appearance. Serum cancer antigen 19-9 levels
decreased from 1,500 U/mL to 404U/mL at 30 days and 407 U/mL at 90 days after
the ablation procedure. Positron emission tomography (PET)/CT imaging
(Fig
5) was
performed 3 months after diagnosis and demonstrated a mild peripheral ring of fluorodeoxyglucose uptake. Although there was no evidence of
residual tumor or nodal disease, a 1.5-cm liver metastasis was also identified on
the 3-month PET/CT scan. Liver metastasis was
treated successfully with percutaneous RF ablation because the lesion was
isolated from large vasculature. Chemotherapy with gemcitabine was then
initiated. Two months after RF ablation, and 6 months after the diagnosis, MR
imaging of the abdomen demonstrated no evidence of disease progression or
recurrence. The cancer antigen 19-9 level decreased to 236 U/mL at 6 months.
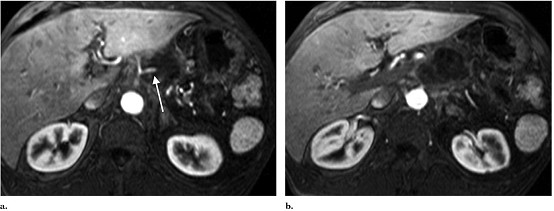
Figure 4. MR image at 1 month
after IRE procedure. (a) Superior aspect of tumor
shows no residual enhancement of tumor, with maintained patency and appearance
of the splenic artery (arrow). (b) At the level of the superior
mesenteric artery, complete necrosis is also seen.
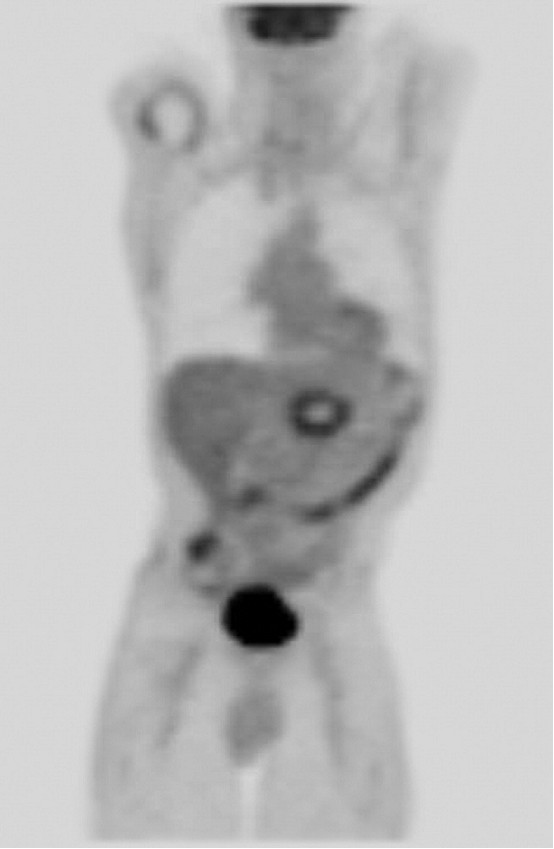
Figure 5. PET/CT image at 3
months. Smooth marginal uptake is seen, which is an expected finding after
ablation. There is no focal residual disease in the pancreatic bed. Focal left
hepatic uptake is not well seen on
coronal projection.
Prev: 納米刀技術在紐約州立大學石溪分校的應用
Next: 肝門附近的轉移瘤的不可逆電穿孔


 Follow on Facebook
Follow on Facebook Follow on Twitter
Follow on Twitter Subscribe to RSS
Subscribe to RSS