Irreversible Electroporation for Focal Ablation at the Porta Hepatis
We treated a 61-year-old man with T3N1M1 moderately differentiated sigmoid adenocarcinoma cancer with multiple liver metastases previously treated with six cycles of neoadjuvant FOLFOX chemotherapy (folinic acid, fluorouracil, oxaliplatin), followed by left hemicolectomy. He subsequently underwent right portal vein embolization followed 8 weeks later by right hepatectomy with extension into segment IVa and caudate lobectomy.
Nine months after surgery, computed tomography (CT) revealed a recurrent liver metastasis at the resection margin in the segment IV remnant (Fig. 1), which lead to treatment in the PICCOLO chemotherapy trial (ClinicalTrials.gov identifier NCT00389870). However, because of his progressive disease , he was referred for percutaneous focal ablation by a multidisciplinary team. CT 1 month before the procedure demonstrated this lesion to now measure 28*9*19 mm. It was recognized that conventional RFA carried the potential for thermal injury to the abutting portal vein and an adjacent small bowel loop. IRE was thus recommended as an alternative.
After obtaining informed consent for treatment, we proceeded with ultrasound-guided IRE (Nanoknife;AngioDynamics,USA) with the patient under general anaesthesia with neuromuscular blockade to avoid unwanted skeletal muscle contraction. Anesthesia was induced with intravenous doses of fentanyl and propofol, then maintained with inhaled isoflurane. Skeletal muscle relaxation was induced before tracheal intubation and maintained with boluses of atracurium.
The lesion and the adjacent portal vein were defined by color Doppler and dynamic contrast-enhanced ultrasound (DCE-US) with a 2-ml intravenous bolus of SonoVue (Bracco,Italy) and a Phillips IU-22 ultrasound platform (Bothell,USA) with a low mechanical index of 0.06.
Before treatment, the tumor measured 28*9*19 mm and was isoenhancing in arterial phase but hypoenhancing in portal and late phase. Tumor dimensions were entered into the built-in treatment planning software and a 10-mm margin selected. A single 18F bipolar electrode was placed into the center of the tumor under ultrasound guidance.
Electrical current was administered in pulses (Table 1) with electrocardiographic synchronization to reduce the risk of cardiac arrhythmia . Current delivery took place
over 10 min. There were no periprocedural adverse events.
At the end of ablation, the treated lesion appeared devascularized; however, unlike with RFA, there was persistence of circulation through small vessels (~1 mm in diameter) passing through the ablation zone on DCE-US (Fig. 2).
The patient was discharged after overnight observation without needing analgesia. At 3 months, there was a decrease in maximum tumor dimensions to 20*17 mm on CT (stable disease according to the response evaluation criteria in solid tumors) (Fig. 3). This corresponded to a decrease in tumor volume size from 5.25 to 3.16 cm3. The patient remains under surveillance.
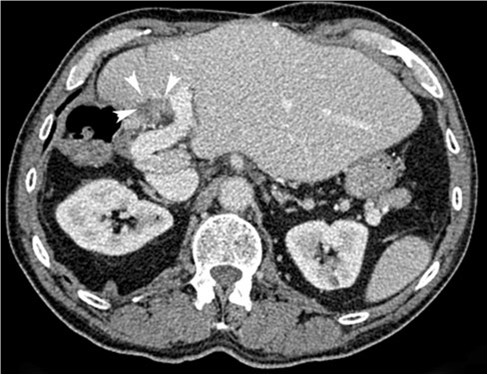
Fig. 1
Pretreatment axial contrast enhanced CT (portal phase) image demonstrating the target lesion (white
arrowheads) abutting the portal vein and an adjacent bowel loop
Table 1 Technical details
|
Characteristic |
Value |
|
Tumor area |
28 *19 mm |
|
Treatment margin |
10 mm |
|
No. of electrodes |
1 |
|
Type of electrode |
Bipolar |
|
French gauge |
18 |
|
Electrode length |
150 mm |
|
Voltage |
2700 V |
|
Pulse length |
70 ms |
|
Pulses per cycle |
90 |
|
No. of cycles |
90 |
|
Ablation time |
10 min |
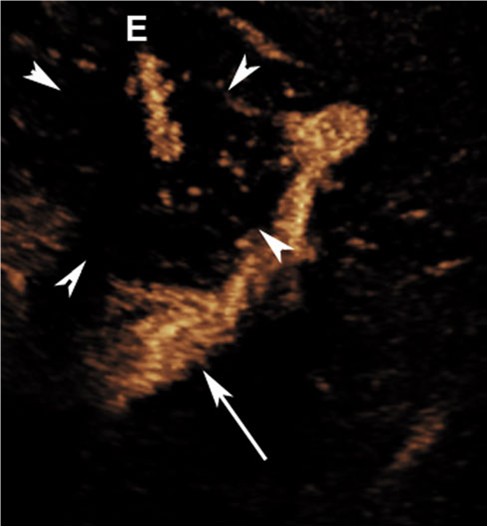
Fig. 2 Postablation axial dynamic contrast-enhanced ultrasound image demonstrating needle (E) within devascularized tumor (white arrowheads). Moving microspheres (yellow dots) within the tumor demonstrate residual blood vessels[1 mm. The adjacent portal vein is seen deep to the lesion (white arrow) (Color figure online)
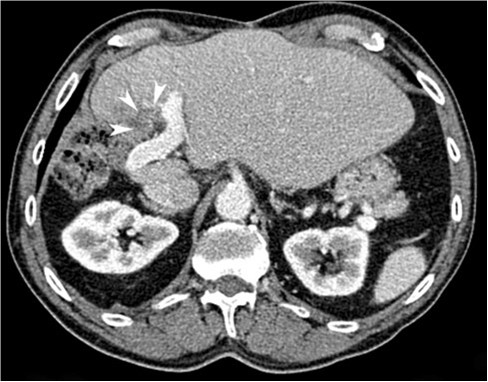
Fig. 3 Follow-up axial CT image (portal phase) demonstrating stable disease
摘自:Irreversible Electroporation for Focal Ablation at the Porta Hepatis
Prev: 病例報告:無法手術切除的胰腺癌的經皮不可逆電穿孔


 Follow on Facebook
Follow on Facebook Follow on Twitter
Follow on Twitter Subscribe to RSS
Subscribe to RSS